Advances in the Study of Bronchial and Vascular Architecture of Lungs in the Rat’s Model: from Morphogenesis to Disease Modelling
bDepartment of Pharmacognosy of Azerbaijan Medical University, Baku, Azerbaijan,
cDepartment of Human Anatomy and Medical Terminology of Azerbaijan Medical University, Baku, Azerbaijan
Keywords
Abstract
Bronchial and vascular architecture in the rat lung forms an interdependent scaffold that balances ventilation with perfusion and adapts to metabolic demand. Development proceeds through coordinated branching programs that couple epithelial growth with vascular patterning while matrix remodeling and epithelial–mesenchymal crosstalk shape airway caliber and capillary alignment. Quantification has moved from classical design-based stereology to organ-scale µCT, optical clearing, and multiscale computational reconstructions that link structure to function. Across disease models, Chronic Obstructive Pulmonary Disease (COPD) and emphysema show distal airspace enlargement with vascular rarefaction, pulmonary hypertension (PH) features medial thickening and arteriolar muscularization, asthma combines epithelial remodeling with angiogenesis, and fibrosis exhibits collagen deposition with capillary regression. Convergent signaling networks integrate these changes, including VEGF and HIF pathways that govern angiogenesis, Notch and Wnt programs that regulate morphogenesis, and oxidative stress with cytokine and microRNA axes that drive vascular remodeling. Translational alignment is strengthened by single-cell and imaging biomarkers that map rat phenotypes to human pathology, while bioengineered platforms and in silico models provide controllable test beds for hypothesis testing. Predictive frameworks for remodeling across development and disease could be provided by standardized pipelines that combine morphometry, mechanics, and molecular profiles.
Introduction
The bronchial and vascular system of the lung ensures even ventilation and perfusion of the parenchyma [1]. Blood oxygenation occurs in the alveolar-capillary interface pulmonary vascular network, and the airflow is controlled by the bronchial tree [2]. Their interdependence in structure promotes effective gas exchange and adaptation to metabolic needs. The close spatial relationship between airway, vascular, lymphatic, and neural networks has also been demonstrated using high-resolution 3D imaging, highlighting the role of architectural integrity in promoting physiological resilience [3]. The study of both development and disease in mammalian lungs is based upon an understanding of these coupled systems.
The FGF10 FGFR2b signaling pathway is required to form lung airways, which is coordinated by biochemical cues and epithelial dynamics to form the hierarchical branching geometry that characterizes lung geometry [4]. The development of the vascular system occurs concurrently via endothelial-mesenchymal signaling, which guarantees the alignment with the airway tree [5]. Mechanistic theories, such as curvature-feedback through ERK, explain how the length and orientation of branches are regulated to achieve morphogenetic homeostasis, with comparative studies revealing that morphogenetic programs are adjusted to functional demands in different organs [6, 7].
The rat has been found to be a useful intermediate model due to its lung size, vascular structure, and hemodynamic accessibility; it has benefits over mice and retains experimental viability [8]. Postnatal µCT imaging indicates persistent alveolar and acinar remodeling, which allows better evaluation of developmental changes [9]. Its clinical value is highlighted by structural changes in heart failure and pulmonary hypertension [10]. The visualization of airway and vascular architecture is now done at high resolution with advanced μCT protocols and CT-based mapping [11, 12].
The recent innovations in stereology have enhanced the measurement of the lung compartments and have made the heterogeneous lesions to be sampled with precision [13]. The ex vivo lung perfusion is relevant to increase the physiological significance of structural evaluation [14]. Whole lung visualization is now achieved at the micron level using the techniques of tissue-clearing and 3D microscopy [15], and high-resolution histological reconstructions that consider the information about gene expression and spatial organization provide new information about the biological process [16]. All these innovations have transformed structural mapping of the rat lung and made it more useful in developmental and disease modeling.
Even though there are significant improvements, there are still gaps in knowledge. The majority of developmental research is based on mice, and rat data are still scarce, even though they are more physiologically similar to humans [17]. The research on airway or vascular remodeling is frequently studied in isolation from each other as opposed to coordinated structural systems [18]. Perfusion pressures and contrast techniques influence distal vascular accuracy, whereas 3DCT and tissue clearing have enhanced visualization [19, 20]. The heterogeneous lesions might be undersampled during the stereological analyses, and thus there is a need to use adaptive sampling and standardised imaging protocols [19]. As a result, there is no single synthesis of morphogenesis, structural mapping, and disease remodeling of rat lungs.
The review is a synthesis of bronchial and vascular architecture of the rat lung with focus on developmental organization, structural interdependence, and pathological remodeling. It combines morphometric, computational, and advanced imaging methods, such as µCT, optical clearing, and corrosion casting, to address discrepancies and explain the shortcomings of methods. Consolidation of the structural baseline can increase the reproducibility, increase comparisons between developmental and disease states, and better translational modeling of airway-vascular coupling. The review offers a consistent framework to answer the major conceptual and methodological gaps in the existing rat lung studies by integrating insights on normal morphogenesis and disease-related remodeling.
The review was created with the help of a narrow literature search of PubMed, Scopus, and Web of Science databases during the period between 2015 and 2025. General search combinations were used in identifying relevant studies, based on rat lung development, bronchial and vascular architecture, imaging modalities, molecular regulation, and experimental disease models. Articles that used rat lungs in imaging studies, translational experimental reports, and other structurally or mechanistically focused studies were selected, and those that lacked structural or mechanistic relevance were excluded. Citation tracking of important publications was used to add more sources. This method guaranteed the full coverage of developmental, imaging-based, molecular, and pathological factors of rat pulmonary architecture.
Developmental Morphogenesis of Rat Lung Architecture
Embryonic and Postnatal Lung Development
Rats pass through the pseudoglandular, canalicular, saccular, and alveolar phases of lung development,
whereby the vascular and bronchial systems develop simultaneously to create a highly branched respiratory
network. Bifurcation of airways starts at the lung buds and continues with the growing vascular plexus,
which eventually develops into a hierarchical arteriovenous system [21]. Though the imaging techniques
like µCT give insight into development, the fundamental process is the coordinated growth of bronchial
branches and vascular structures that allow efficient respiratory architecture [22]. Models of
cross-species organoid and pluripotent stem cells indicate that cardiopulmonary co-development is
conserved, with epithelial and endothelial differentiation having to proceed in parallel to reach
functional maturation [23]. VEGF is a key mediator of vascular bed development, and VEGF signaling defects
have great consequences on alveolarization and vessel branching [24, 25]. VEGF deficiency or hypoxic
stress impedes the development of the distal vessels' arborization and septation, which supports the
dependence of both epithelial and vascular growth in morphogenesis [26]. Branching of the airways involves
the involvement of fibroblast growth factor 10 (FGF10) and its receptor FGFR2b, and the change in these
signals can change vascular alignment indirectly by regulating epithelial geometry [27]. In prenatal and
postnatal stages, epithelial-mesenchymal crosstalk is proportionately controlled by signaling pathways,
including SHH, WNT, and TGF-β [28, 29]. Notch-dependent signaling also enhances airway and vascular
patterning through the preservation of coherent growth and the regulation of epithelial cell fate [30].
Integrative studies reveal that airway branching and vascular patterning are mutually instructive
processes, with endothelial cues regulating airway topology and vice versa [31-34]. Collectively, these
processes suggest that the morphogenesis of the rat lung is a highly coordinated process that is
influenced by molecular gradients, juxtacrine signaling, and mechanical forces.
Cellular and Extracellular Matrix Dynamics
Morphogenesis of rat lungs involves the maintenance of constant contact between the mesenchymal and
epithelial cells that form future bronchial and vascular tissues. Epithelial-mesenchymal interactions
control the smooth muscle investment, angle of branching, and differentiation based on the reciprocity of
a growth factor, matrix remodeling, and mechanical tension signaling loops [35, 36]. The extracellular
matrix (ECM) is a structural scaffold and a signaling platform that promotes alveolarization and septation
[37]. The adhesion between epithelial stability and basement membrane integrity is mediated by integrin
and ensures structural maturation and controls inflammatory homeostasis [38]. The stabilization of
developing vessels by paracrine signals of pericytes and fibroblasts, the dynamic response of endothelial
cells to matrix stiffness and architecture, controls capillary sprouting and arteriovenous differentiation
[39]. The ECM, which consists of collagens, elastin, fibronectin, and laminin, develops during both
prenatal and postnatal stages to assist in alveolar recoil characteristics and growth, which is necessary
for effective ventilation [40]. On the whole, the ECM dynamics combine with the epithelial and mesenchymal
signaling to develop mechanically stable but flexible airway and vascular systems.
The results of rat development should be viewed with caution since the variation in the pattern of
branching and vascular alignment between strains provides variability in studies [22]. Also, integrative
analyses show that epithelial-endothelial coordination is very context-dependent and thus observations
made with one rat strain cannot necessarily be reliably generalized to other species or experimental
conditions [31].
Coordinated airway and vascular development during different embryonic and postnatal development stages
determines rat lung morphogenesis, which is regulated by reciprocal epithelial-endothelial signaling and
highly controlled molecular pathways that determine patterns of branching and vascular alignment.
Mechanobiological cues, the extracellular matrix, also contribute to structural support and guide
maturation of the alveoli and vascular. The combination of these processes of development forms the
primary bronchial-vascular architecture that forms the basis of subsequent functional performance and
remodeling pathology.
Imaging and Quantitative Approaches
Classical Histomorphometry and Stereology
Histomorphometric and stereological methods have long been used in the quantitative evaluation of lung
structure, which offers objective and reproducible estimates of airway and vascular parameters of fixed
tissue specimens. To close the divide between architectural design and gas-exchange efficiency, first,
stereological models were created to measure the alveolar volume, surface area, and capillary density
[41]. Design-based stereology is still a major method used in the measurement of microvascular and
bronchial architecture of rat lungs and can be reproducibly used in both developmental and pathological
models. The finesse of the stereological concepts of rodents has now allowed the detailed description of
the microvascular branching, the thickness of the vessel walls, and the size of the bronchial lumen in
physiological and disease states [42]. Stereology, when used together with systematic uniform random
sampling, removes the distortions of two-dimensional histology and provides statistically representative
estimates of three-dimensional quantities [43]. In order to improve precision between tissue hierarchies,
recent methods combine computed tomography with histological sections to produce multiscale datasets to be
used in stereological computation [44]. Stereology remains a valid method of measuring vascular
rarefaction, interstitial expansion, and bronchial remodeling in experimental rat preparations and normal
lung tissue [45]. Modern multiresolution workflows can be used to take the classical stereological
paradigm and transform it into a single system of analysis that allows hierarchical measurements across
scales by using macroscopic volumetric data as well as microscale quantification [46]. Even though
classical stereology is still destructive, it is still regarded as the gold standard of volumetric
calibration of digital imaging results in rat morphometric studies [47].
Modern 3D and In vivo Imaging
Recent advances in technology have changed the two-dimensional histology of the lungs into a
three-dimensional volumetric analysis of intact rat lungs, which is dynamic. The micro-computed tomography
(Micro-CT or µCT) enables the determination of airway diameter, vessel density, and branching geometry at
a resolution of micrometers across entire volumes of lungs [48]. Precise reconstruction of the
microvasculature of the lungs can be obtained in case of proper control of perfusion pressures and
contrast enhancement, and almost native visualization of the arterial and venous hierarchies can be
achieved [49]. Optical clearing and light-sheet microscopy can be used as a complement to µCT, allowing
mapping microvascular-bronchial relationships at a fine scale in transparent, fluorescently stained rat
lungs [50]. Improvements in aerosol-based clearing techniques currently allow longitudinal imaging of the
inflammatory and infectious events in vivo with enhanced imaging penetration and temporal
resolution [51]. Multiscale three-dimensional imaging systems combine both the macrovascular and
microvascular data to produce organ-scale views of the bronchial and vascular hierarchies [52].
Computational modeling also improves such datasets through digital reconstruction of vascular networks and
bronchial patterns of branching, which allows simulation of the interactions between hemodynamics and
ventilatory conditions relevant to physiology [53]. These virtual lung models accurately recreate in
vivo mechanical conditions with experimentally obtained values of pulmonary volume, pressure, and
strain [54]. In fetal morphometry and developmental toxicology, it has been shown that volumetric imaging
and µCT are capable of detecting small changes in airway and vascular development, which can be used to
give quantitative measures of translational development [55]. High-resolution imaging, optical clearing,
and computational modeling are a complete paradigm that brings together structural quantification, spatial
organization, and predictive simulation.
Imaging and stereological techniques, although positive, have significant drawbacks. Stereological
estimates are also prone to sampling strategy and inflation bias, which may create systematic variability
among laboratories [43]. Perfusion pressure and uniformity of contrast are the key factors affecting the
accuracy of µCT, which leads to inconsistent visualization of distal vessels across studies [49].
Stereology and µCT are the gold standard of volumetric and vascular reconstruction accuracy, and
quantitative imaging modalities differ in the resolution of analysis at the morphometric scales (Table 1).
Fig. 1 represents the workflow of the imaging and quantitative methods that were employed to analyze the
morphometry of rat lungs. Fig. 2 [53] illustrates an example of high-resolution whole-lung reconstruction
of a µCT of bronchial vascular architecture of intact rat lungs. The left panel is the horizontal slice of
µCT that illustrates the global airway and parenchymal architecture of the rat lung, and the right one is
the magnified view of the boxed area, which represents the detailed microstructure of the alveoli and
acinar.
Multi-scale reconstruction of rat bronchial and vascular architecture can be done using imaging and
quantitative methods - classical stereology to µCT and light-sheet microscopy. The gold-standard
validation framework is provided by stereology, and the high-resolution structural mapping of organs in a
comprehensive manner is offered by modern 3D imaging. Computational modeling goes a step further to
combine these datasets and simulate physiological interactions, and improve interpretability. These
instruments are collectively a consistent system of quantitative tools necessary to research development,
remodeling, and disease in rat lungs.
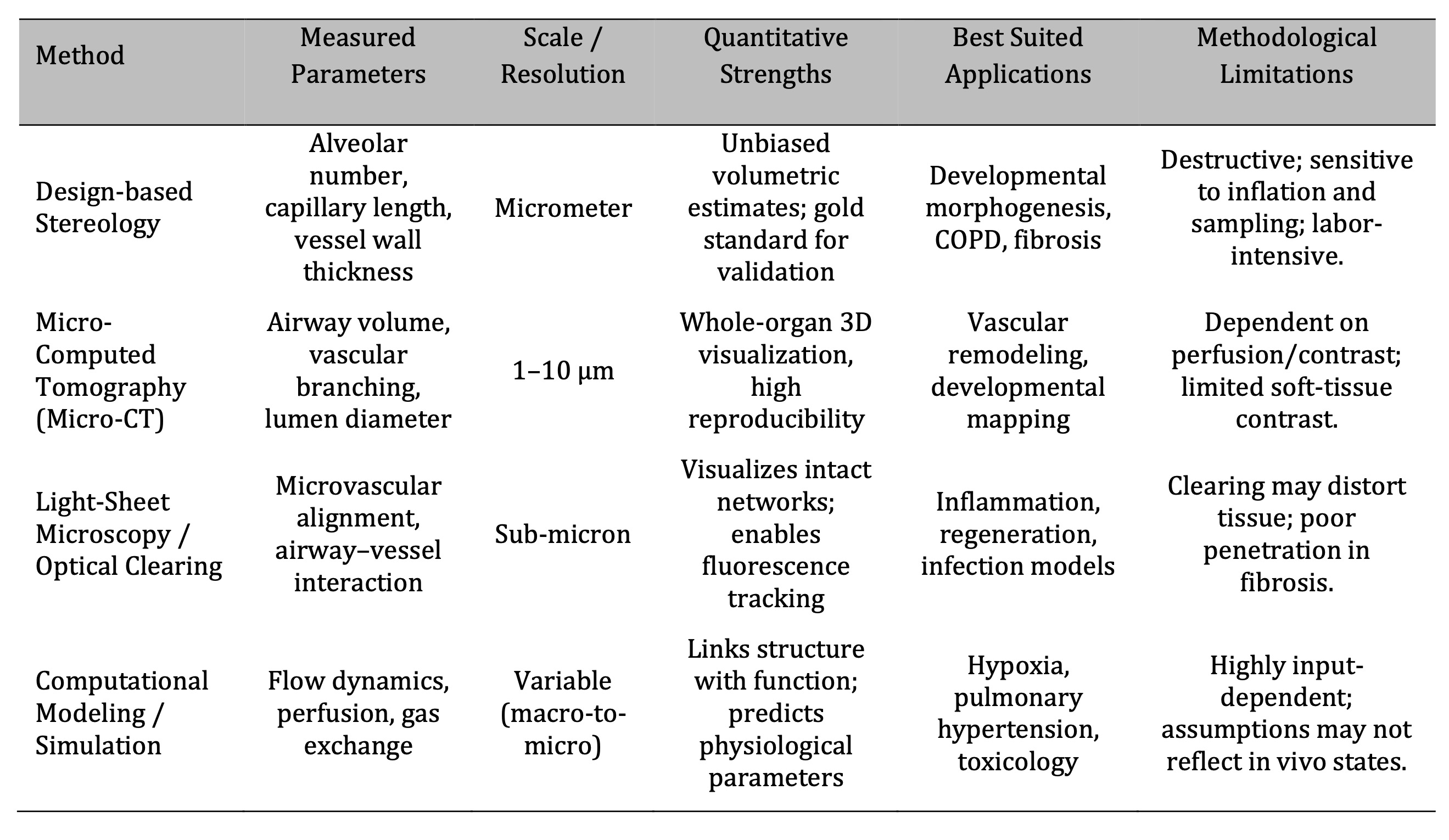
Table 1: Comparative Overview of Quantitative Imaging and Analytical Techniques in Rat Lung Architecture Studies
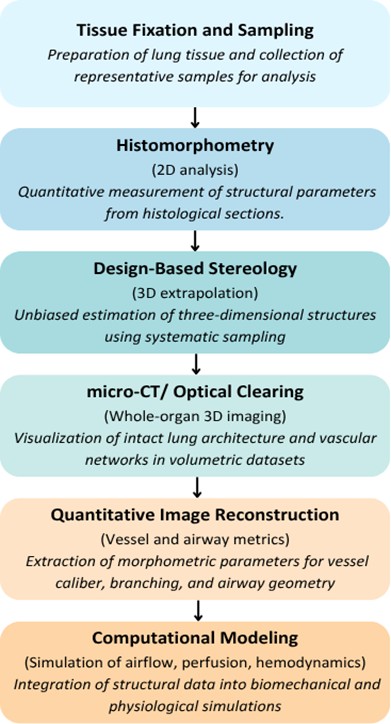
Fig. 1: Workflow of Imaging and Quantitative Approaches in Rat Lung Morphometry.
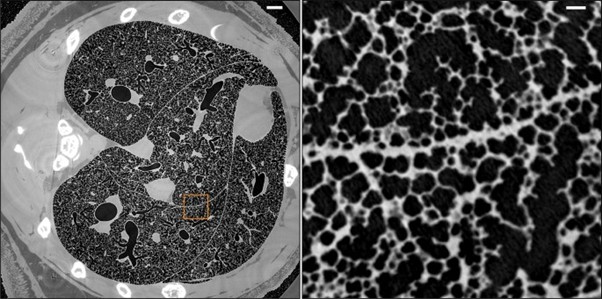
Fig. 2: Micrometer-resolution X-ray micro-CT of an intact post-mortem juvenile rat lung (reproduced from ref. [53], under CC BY 4.0 license).
Experimental Models of Lung Diseases in Rats
Models of Chronic Obstructive Pulmonary Disease (COPD) and Emphysema
The analysis of structural and vascular alterations underlying progressive airflow limitation with the
help of experimental rat models of emphysema and chronic obstructive pulmonary disease (COPD) has been
critical. Human COPD is characterized by the destruction of the alveoli and small airway remodeling, which
are recapitulated by the classical induction methods, including cigarette smoke or proteolytic agent
exposure [56]. These rats exhibit the progressive emphysematous changes, which, according to longitudinal
modeling, include the quantifiable loss of parenchymal elasticity and capillary rarefaction, which follow
the progression of human disease [57]. Morphometric studies demonstrate that there is an increase in the
size of distal airspaces, alveolar septa become thin, and the surface area of capillaries is reduced,
which increases dead space and reduces the efficiency of gas-exchange [58]. Injury in neonatal and
juvenile rats caused by hyperoxia induces the same structural continuum, alveolar simplification, and
vascular rarefaction occurring concurrently [59]. Combined COPD-cor pulmonale models also enable
concurrent evaluation of right ventricular adaptation and pulmonary vascular remodeling, which enhances
the structure-function relationships with chronic airflow limitation [60]. The characteristic
architectural alterations, including thickening of the small airway walls, loss of the distal vascular
density, and altered smooth-muscle organization, which are highly reminiscent of airway-vascular coupling
disruptions seen in human COPD, are also identified in smoke-exposure models [61]. Taken together, these
rat models are a good representation of the morphometric and hemodynamic characteristics of COPD, which
allows interventions to be controlled to protect airways and vascular integrity.
Pulmonary Hypertension and Vascular Remodeling
The rat model of pulmonary hypertension (PH) is a well-defined model for studying vascular structural
remodeling. Chronic hypoxia or chemical agents like monocrotaline or Sugen cause prolonged increases in
pulmonary arterial pressure, which in turn leads to the medial hypertrophy, adventitial thickening, and
muscularization of the distal arteries [62]. The reversal of neointimal proliferation by therapeutic
studies such as paclitaxel-based interventions demonstrates the structural reversal of neointimal
proliferation, which highlights the usefulness of PH models in testing anti-remodeling strategies [63].
The Sugen-hypoxia model allows cardiac magnetic resonance imaging to be used to allow longitudinal
evaluation of biventricular structural and functional responses, correlating right-heart responses with
pulmonary vascular load [64]. Despite the fact that this model mainly reflects severe pulmonary arterial
hypertension, related parenchymal injury, and mild patterns of emphysema indicates the structural
interaction between vascular and airway compartments in the advanced disease [65]. Collectively, these PH
models offer critical information about the thickening of the vascular, the stiffening of the vessel, and
the hierarchical remodeling of the pulmonary arterial tree.
Asthma and Inflammatory Models
Rat models of asthma and allergic airway inflammation are the focus of structural interaction between
immune activation, angiogenesis, and airway remodeling. Ovalbumin or house-dust-mite antigen sensitization
results in reproducible airway hyperresponsiveness that is associated with vascular proliferation and
deposition of extracellular matrix in the peribronchial region [66]. Histologically, neovascularization,
goblet-cell hyperplasia, and epithelial basement-membrane thickening are always evident, and they
represent the organized remodeling of vascular, mesenchymal, and epithelial compartments [67].
Interventional models of allergic inflammation indicate that structural outcomes, including decreased
peribronchial vessel density or decreased smooth-muscle thickening, can be measured quantitatively in
these models, which validates their usefulness in the testing of remodeling-directed therapies [68]. The
models of asthma also demonstrate the presence of expanded bronchial vascular plexus and the change in
vessel permeability that leads to the thickening of the airway wall and the narrowing of the lumen [69].
Taken together, these inflammatory models offer a reproducible model of evaluation of airway and vascular
remodeling in an allergic state.
Fibrosis and Acute Lung Injury
Experimental models of fibrosis and acute lung injury are essential for understanding the structural
distortion and reparative vascular responses that are related to chronic lung disease. Activation of
macrophages, cytokine release, and matrix deposition in response to exposure to toxicants or hypoxia is
highly reminiscent of human interstitial fibrosis [70]. Experiments of smoke-induced injury indicate that
corticosteroid timing and dose affect vascular remodeling, collagen turnover, and final fibrotic
phenotype, highlighting the structural plasticity of injured lung tissue [71]. Models based on bleomycin
are still the gold standard as they offer quantitative data of anti-fibrotic activity and architectural
recovery [72]. The association between oxidative stress, vascular leakage, and endothelial barrier
disruption is further demonstrated using ischemia-reperfusion injury models, which can be prevented
through antioxidant therapy, including edaravone [73]. Natural and synthetic interventions, such as
resveratrol nano-capsules and crocin, have been demonstrated to inhibit fibrosis, inflammation, and
vascular dysfunction, which contributes to their possible therapeutic significance [74, 75]. These models
taken together describe the cascade of events of epithelial injury, vascular repair, and matrix remodeling
that control chronic fibrotic progression.
Despite the numerous structural parallels of COPD and fibrosis, rat disease models are based on induced
injuries, which may not completely recapitulate the heterogeneous, slow progression of human disease [56].
Equally, fibrosis induced by bleomycin causes homogenous parenchymal damage, unlike the focal and
heterogeneous injury in the clinical presentation [72].
In structural remodeling, several of the conserved signalling cascades integrate fibrotic, inflammatory,
and angiogenesis (Table 2), reflecting the molecular interdependency of the vascular and bronchial
systems. Fig. 3 demonstrates the grouping of experimental rat models and the structural changes that occur
in them.
Rat models of COPD, pulmonary hypertension, asthma, and fibrosis reproduce specific patterns of airway and
vascular remodeling that are highly similar to human disease. Their structural effects, such as
destruction of alveoli, vascular rarefaction, arterial thickening, neovascularization, and matrix
deposition, allow accurate morphometric evaluation in disease conditions. Architectural variations of the
models are also effective in offering a solid platform for assessing treatments that focus on airway and
vascular integrity. These experimental systems as a whole constitute a complete structural toolkit to
study pathological remodeling in the rat lung.
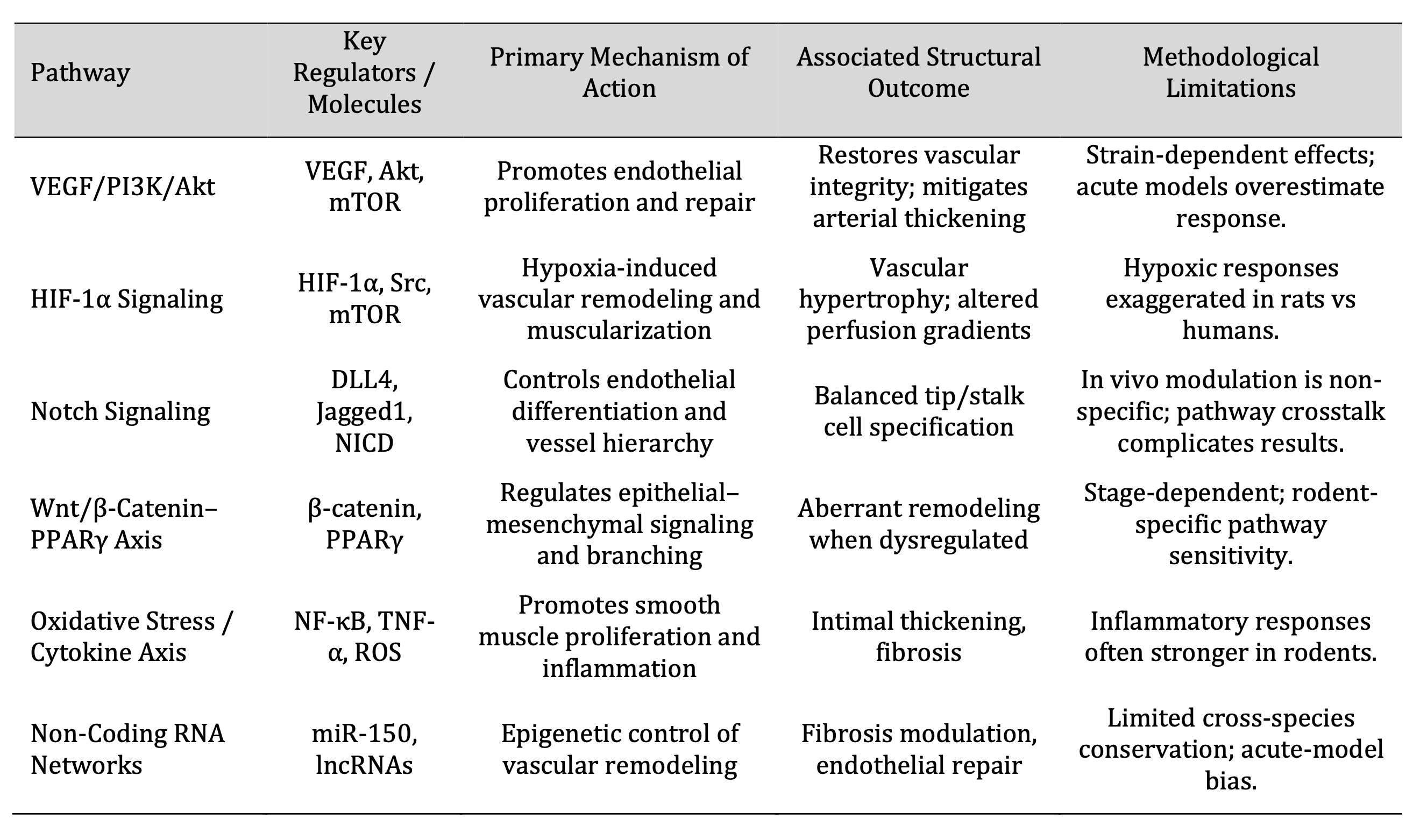
Table 2: Key Molecular Pathways and Signaling Axes Governing Pulmonary Architectural Remodeling in Rats
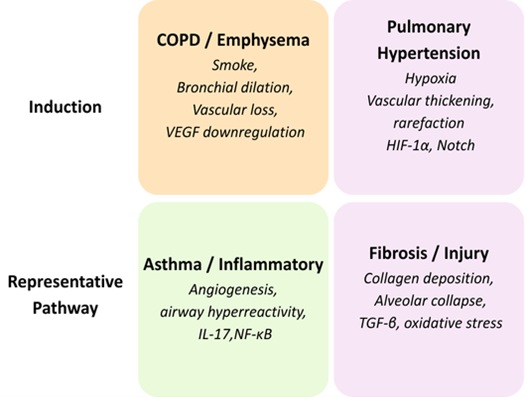
Fig. 3: Classification of Experimental Rat Models and Associated Structural Alterations.
Molecular Pathways Governing Architectural Remodeling
Key Angiogenic and Morphogenetic Pathways
In the rat lung, morphogenesis of the bronchial and vascular systems is tightly controlled by conserved
molecular pathways incorporating hypoxic, angiogenic, and developmental cues. Notch signaling is a key
orchestrator of endothelial specification and vascular hierarchy, in which it keeps the tip-stalk cell
differentiation balanced in sprouting angiogenesis [76]. VEGF/PI3K/Akt cascade activation restores the
endothelial functional activity and decreases pulmonary arterial thickening in COPD-induced vascular
remodeling, which is associated with metabolic control and angiogenic competence [77]. Hypoxia-inducible
factor-1α (HIF-1α) pathway is an oxygen-sensing regulator that links hypoxic conditions to vascular
growth, and its malfunctioning leads to the excessive muscularization of pulmonary hypertension [78].
Endothelial-derived angiocrine factors also influence epithelial branching and alveolar maturation,
highlighting reciprocal vascular–airway communication during development and repair [79]. Interactions
between PPARγ and Wnt/β-catenin signaling guide epithelial differentiation and vascular alignment, and
imbalances among these networks contribute to pathological architectural remodeling [80]. VEGF, HIF-1α,
Notch, and Wnt signaling constitute an integrated regulatory axis that regulates angiogenesis,
epithelial-vascular interactions, and tissue homeostasis in normal and disease lung [81].
Inflammatory and Oxidative Mechanisms in Vascular Remodeling
Redox-responsive pathways and reactive oxygen species (ROS) are significant regulators of inflammatory and
structural remodeling in rat models of pulmonary disease. Mitochondrial dysfunction, endothelial
apoptosis, and perivascular inflammation are sustained by prolonged oxidative stress in pulmonary
hypertension and remodel the vascular wall [82]. Chronic redox imbalance may trigger
endothelial-to-mesenchymal transition, leading to adventitial fibrosis and microvascular obliteration,
which resembles human pulmonary pathology [83]. Inflammatory cytokine activation—particularly within the
NF-κB/TNF-α axis—exacerbates vascular injury and promotes smooth-muscle hypertrophy and intimal thickening
in hypoxia-induced models [84]. Chronic hypoxia induces the upregulation of microRNA-150 that suppresses
vascular remodeling through inhibiting profibrotic and inflammatory cascades, which rejuvenate endothelial
functions and pulmonary hemodynamics [85]. All these processes indicate that there is close molecular
interaction between oxidative stress, cytokine release, and regulation by microRNAs in adaptive and
maladaptive remodeling of the pulmonary vasculature.
Genetic and Epigenetic Regulation of Remodeling
Genetic and epigenetic changes are increasingly recognized as key determinants in the transition from
reversible injury to chronic pulmonary remodeling. Histone acetylation and methylation control
transcriptional reactions to oxidative injury and vascular pathology, and environmental stressors promote
dynamic histone chromatin remodeling in rat models of fibrosis and pulmonary hypertension [86]. Long
non-coding RNAs and microRNAs have been shown to regulate vascular contractility and endothelial
differentiation, and experimental manipulation of these RNAs has been shown to change the course of
disease in rat pulmonary arterial hypertension models [87]. In chronic thromboembolic pulmonary
hypertension, transcriptomic studies indicate that the patterns of gene-expression differences that
regulate extracellular-matrix turnover, angiogenesis, and inflammation are heritable reprogramming of
vascular and interstitial cell fate [88]. Fibroblast reprogramming studies of rodent tissues have shown
that intermediate trans-endothelial-like states can increase reparative capacity, which can inform us
about the mechanisms underlying structural regeneration [89]. Chromatin data of the rat parenchymal
disease models on a genome-wide scale also show reproducible epigenetic signatures of vascular distortion
and fibrotic development [90]. Taken together, these results indicate that genetic, epigenetic, and
transcriptional regulators combine with environmental and molecular signals to determine the pathway of
pulmonary architectural remodeling.
Although rat models have helped to elucidate many signaling pathways, there are still a number of
translational differences. Hypoxia-regulated HIF-1α signaling differs in magnitude between species,
influencing vascular-proliferative responses [78], while inflammatory pathways such as NF-κB/TNF-α
activation may be exaggerated in rodent models relative to chronic human disease [84].
Angiogenic, inflammatory, and fibrotic responses are interconnected through several conserved signaling
cascades in the process of structural remodeling (Table 3), which indicates the molecular interdependence
of bronchial and vascular systems.
Key molecular pathways—including VEGF, HIF-1α, Notch, and Wnt/β-catenin—form a coordinated regulatory axis
controlling angiogenesis and airway–vascular alignment. Maladaptive vascular remodeling is also further
promoted by oxidative stress, cytokine signaling, and microRNA networks in various models of rat disease.
Long-term changes in vascular and interstitial cell fate are determined by genetic and epigenetic
regulators, including chromatin modulations and non-coding RNAs. A combination of these molecular systems
describes the convergence of the inflammatory, angiogenic, and fibrotic responses to generate the
structural remodeling.
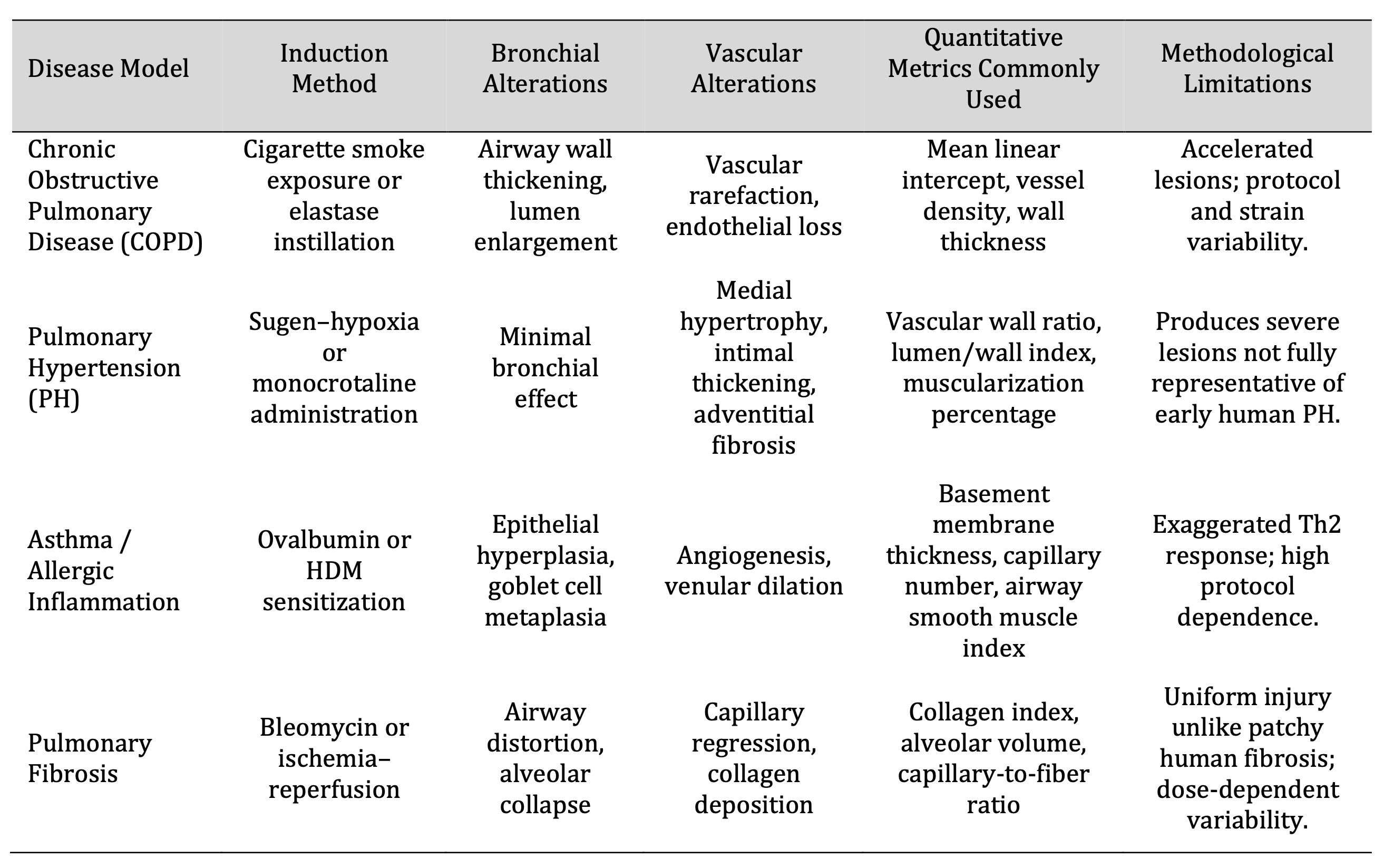
Table 3: Structural Remodeling Patterns Across Experimental Rat Models of Lung Disease
Integrative Translational Perspectives
Translational Relevance of Rat Pulmonary Architecture
The rat lung model has significant translational potential and offers a mechanistic understanding of
clinical importance because it is closely physiologically similar to the human pulmonary system. The
analysis of the single-cell gene expression and remodeling in the rat lungs is comparable to the results
of human pulmonary arterial hypertension, showing that there is a shared vascular pathobiology [91].
Translational fidelity is also confirmed with aerosol-based inhalation studies, whereby the dynamics of
droplet transport, deposition, and clearance scales between rat and human airway geometries can be
predicted [92, 93]. Precision-cut lung slices have demonstrated that rat pulmonary tissue mimics human
xenobiotic enzymatic profiles and thus can be used in preclinical drug-safety testing [94]. Also,
multifaceted models of pulmonary hypertension and right-ventricular remodeling in rats recapitulate
experimentally determined hemodynamic and architectural patterns, which can be used to extrapolate
therapeutic targets and strategies [95]. Taken together, this establishes that the rat offers a
physiologically healthy platform that connects preclinical mechanistic data to human lung pathophysiology.
Integrating Morphometric and Molecular Frameworks for Precision Modeling
The rat lung has turned out to be a useful platform to combine molecular and structural data, which are
fueled by multimodal imaging and omics technologies. Quantitative morphometric analysis, which is coupled
with high-resolution imaging, provides reproducible spatial measures of airways, vessels, and parenchyma
that are translational biomarkers of emphysema and pulmonary fibrosis [96, 97]. Three-dimensional
reconstruction and optical imaging methods directly observe multiscale structural remodeling and also
record molecular correlates of inflammation and tissue repair [98]. Regional density and vascular
tortuosity are imaging biomarkers that have a strong association with histopathological severity in rat
models and parallel imaging phenotypes in human interstitial lung disease [99]. Computational registration
and cross-species anatomical mapping improve comparative knowledge of bronchiolar and lobular structure in
rodents and humans [100]. Transcriptomic analysis of rat and human airway epithelium also shows that there
is a conserved gene-network regulation of epithelial differentiation and immune responses [101, 102]. A
combination of these integrative approaches combines molecular profiling with quantitative morphometry to
produce reproducible, cross-compatible data sets that enhance translational pulmonary modeling.
Computational Extrapolation and Cross-Species Validation
Computational models are increasingly allowing one to extrapolate rat data to human respiratory states.
Now species-agnostic comparisons of lung cell populations can be made using single-cell atlases and
transcriptomic mapping, which can be used to predict species-conserved vascular-signaling and
matrix-remodeling pathways [103, 104]. Simulations in silico are reliable predictors of particle
transport, dose distribution, and deposition efficiency when flow dynamics and airway geometries of rats
are scaled to human conditions [105]. Combining computational results with morphometric and molecular data
can be used to create digital lung twins that can recreate physiologic behavior across species. The use of
these models in comparison to empirical data of rat and human systems improves algorithms to predict gas
exchange, mechanical stress, and drug-absorption profiles. This integration of computational surrogate and
biological validation makes the rat model a predictive translational system rather than a descriptive
experimental system, and makes it more relevant to accurate respiratory research.
Although there are strong parallels in the translation, species-specific differences do not allow direct
extrapolation of rat-based results. Single-cell studies indicate partial but not complete correspondence
of rat and human vascular signaling networks [91], and xenobiotic metabolism, while broadly similar, still
differs in key enzyme pathways relevant for drug-response prediction [94].
Rat lungs exhibit physiological, metabolic, and structural characteristics that are highly similar to
those of the human lungs, which makes them highly translational. A combination of morphometric data and
molecular and imaging-based analyses would allow cross-species biomarkers of structural lung disease to be
reproducible. Predictions of gas exchange, airflow dynamics, and drug delivery are also enhanced with the
help of computational scaling and digital models of lung twins. Collectively, these strategies make the
rat a strong translational tool between the experimental results and the human pulmonary pathophysiology.
Emerging Technologies in Pulmonary Structural Analysis
Advanced Imaging and Quantitative Modeling
Recent breakthroughs in quantitative modeling and computational imaging have revolutionized the study of
pulmonary structure and vascular remodeling using rat models, where µCT imaging can be used to visualize
microvascular adaptation to hemodynamic or hypoxic stress with high spatial resolution, and the patterns
of remodeling heterogeneity can be used to better understand cardiopulmonary coupling in pulmonary
hypertension [107]. Computational models that take morphometric inputs reproduce airflow distribution,
pressure gradient, and vascular resistance through fluid dynamics and tissue deformation simulation of the
rat lung [108]. Such combined approaches combine both anatomical measurements and functional performance
to improve the accuracy of preclinical disease modeling and therapeutic assessment.
Bioengineered Lung Models and Translational Interfaces
Microengineered bio-platforms have become capable of recreating key rat lung biomechanical and
microvascular physiological features. Lung-on-chip and 3D-printed scaffolds are alveolar-capillary
interfaces re-engineered with microfluidic channels covered with epithelial and endothelial cells, and
allow dynamic modeling of inflammatory, fibrotic, or mechanical stimuli [109]. These devices can
manipulate airflow, perfusion, tissue stretch, and gas-exchange parameters in a controlled manner, usually
based on imaging-based templates of rat lung architecture. These bioengineered systems can be used to
support physiologically relevant testbeds, which can be used to further preclinical validation of
molecular and pharmacologic intervention and translational alignment between human pathology and rat in
vivo experiments.
Integrative Systems Biology and Predictive Analytics
The imaging, molecular, and computational datasets are becoming more and more integrated into systems
biology approaches to produce predictive frameworks of pulmonary remodeling. Data integration using
artificial intelligence links structural, transcriptional, and metabolic networks together, providing a
mechanistic understanding of inflammation, fibrosis, and angiogenesis [110]. These models integrate
multi-omics data with morphometric data to project architectural changes in both development and disease.
The integration of digital morphometry and systems-level models provides a platform of accurate and
multiscale predictions of lung behavior and facilitates the design of interventions.
Future Directions in Technological Integration
Further convergence of systems biology, computational modeling, and state-of-the-art imaging will be
useful in future pulmonary structural studies. Dynamic analysis of the co-evolution of airway branching
and vascular remodeling throughout disease progression will be possible using high-resolution
visualization with biomechanical and molecular data. Creating open-access, standardized morphometric and
multi-omics data repositories will enhance reproducibility and translational applicability between rat
models and human experiments. The combination of digital simulations and bioengineered organ-level systems
into predictive multiscale models is the next step in the analysis of pulmonary structure, which
correlates structure, function, and molecular regulation at never-before-seen levels. The directions in
this field are new and summarized in Fig. 4.
New technologies are still limited to methodological inconsistency, with µCT segmentation and
quantification being reliant on the calibration of scanners and perfusion status [107], and computational
simulations being susceptible to minor morphometric errors, which can cause a considerable change in the
distribution of predicted airflow or pressure [108].
The high-precision analysis of rat lung structure is now possible due to the use of advanced µCT imaging,
computational modeling, and multiscale reconstruction. Physiologic microenvironments, such as lung-on-chip
systems, are bioengineered platforms that are used to improve preclinical modeling and translational
testing. The integration of morphometric and molecular data by systems biology and AI also produces
predictive and cross-scale models of lung remodeling. Further advancements will be based on integrating
digital simulations, high-resolution images, and open-access data to enhance translational relevance.
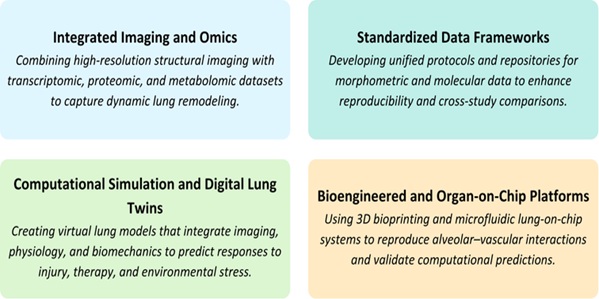
Fig. 4: Future Directions in Pulmonary Structural Research.
Conclusion
The rat lung is an immensely informative model for studying the coordinated architecture of bronchial and vascular systems throughout development and pathology. The development of imaging, stereology, and molecular profiling has enhanced the knowledge of the interaction and remodeling of these compartments in diseases like fibrosis, pulmonary hypertension, and airway injury. The combination of morphometric accuracy with molecular and computational data is now used to improve the predictive and translational usefulness of rat studies. Further advancements in the standardization of imaging, multiscale data analysis, and analytical processes will enhance the usefulness of this model as an essential linkage between experimental discovery and human pulmonary biology.
Acknowledgements
The authors gratefully acknowledge the support of the Department of Cytology, Embryology, and Histology; Department of Pharmacognosy as well as the Department of Human Anatomy and Medical Terminology at Azerbaijan Medical University, Baku, for providing institutional assistance and access to academic resources. The authors also extend their appreciation to colleagues whose insights and constructive discussions contributed to the refinement of this review.
Author Contributions
Aliyarbayova Aygun Aliyar: Conceptualization, Supervision, Writing– original draft, Correspondence;
Aliyeva Sanam Eldar: Literature review, Critical evaluation of studies, Writing –review & editing ;
Shukurova Ayten Sadig: Thematic synthesis, Manuscript organization, Editing. Gasimova Tarana Mubariz:
Conceptual input, Structural refinement, Proofreading; Mustafayeva Nigar Adil: Review of recent
literature, Reference verification, Editing; AliyevaSabina Aydın: Manuscript formatting, Visualization,
Proofreading.
Funding Sources
This review did not receive any specific grant from funding agencies in the public, commercial, or
not-for-profit sectors.
Statement of Ethics
This article is based on previously published studies and does not involve any new experiments with human
participants or animals performed by any of the authors.
Disclosure Statement
The authors declare no conflicts of interest regarding the publication of this review article.
References
| 1 | Mühlfeld C. Stereology and three-dimensional reconstructions to analyze the pulmonary vasculature.
Histochemistry and Cell Biology. 2021 Aug;156(2):83-93.
https://doi.org/10.1007/s00418-021-02013-9 |
| 2 | Hopkins SR, Stickland MK. The pulmonary vasculature. InSeminars in respiratory and critical care
medicine 2023 Oct (Vol. 44, No. 05, pp. 538-554). Thieme Medical Publishers, Inc..
https://doi.org/10.1055/s-0043-1770059 |
| 3 | Zhao S, Cui J, Wang Y, Xu D, Su Y, Ma J, Gong X, Bai W, Wang J, Cao R. Three-dimensional
visualization of the lymphatic, vascular and neural network in rat lung by confocal microscopy.
Journal of Molecular Histology. 2023 Dec;54(6):715-23.
https://doi.org/10.1007/s10735-023-10160-7 |
| 4 | Jones MR, Chong L, Bellusci S. Fgf10/Fgfr2b signaling orchestrates the symphony of molecular,
cellular, and physical processes required for harmonious airway branching morphogenesis. Frontiers
in Cell and Developmental Biology. 2021 Jan 12;8:620667.
https://doi.org/10.3389/fcell.2020.620667 |
| 5 | El Agha E, Iber D, Warburton D. Branching Morphogenesis During Embryonic Lung Development.
Frontiers in Cell and Developmental Biology. 2021 Jul 12;9:728954.
https://doi.org/10.3389/fcell.2021.728954 |
| 6 | Hirashima T, Matsuda M. ERK-mediated curvature feedback regulates branching morphogenesis in lung
epithelial tissue. Current Biology. 2024 Feb 26;34(4):683-96.
https://doi.org/10.1016/j.cub.2023.12.049 |
| 7 | Lang C, Conrad L, Iber D. Organ-specific branching morphogenesis. Frontiers in Cell and
Developmental Biology. 2021 Jun 7;9:671402.
https://doi.org/10.3389/fcell.2021.671402 |
| 8 | Cantor, J. (2025). Rodent Models of Lung Disease: A Road Map for Translational Research.
International Journal of Molecular Sciences, 26(17), 8386.
https://doi.org/10.3390/ijms26178386 |
| 9 | Haberthür D, Yao E, Barré SF, Cremona TP, Tschanz SA, Schittny JC. Pulmonary acini exhibit complex
changes during postnatal rat lung development. PLoS One. 2021 Nov 8;16(11):e0257349.
https://doi.org/10.1371/journal.pone.0257349 |
| 10 | Jasińska-Stroschein M. An updated review of experimental rodent models of pulmonary hypertension
and left heart disease. Frontiers in Pharmacology. 2024 Jan 8;14:1308095.
https://doi.org/10.3389/fphar.2023.1308095 |
| 11 | Napieczyńska, H., Kedziora, S. M., Haase, N., Müller, D. N., Heuser, A., Dechend, R., & Kräker, K.
(2024). μCT imaging of a multi-organ vascular fingerprint in rats. PLoS One, 19(10), e0308601.
https://doi.org/10.1371/journal.pone.0308601 |
| 12 | Rekabdar T, Yousefi M, Masoudifard M, Zehtabvar O, Ahmadpanahi SJ. Computed Tomographic Anatomy
and Topography of the Lower Respiratory System of the Mature Rat (Rattus norvegicus). Archives of
Razi Institute. 2024 Oct 31;79(5):981.
https://doi.org/10.32592/ARI.2024.79.5.981 |
| 13 | Mühlfeld C, Schipke J, Labode J, Ochs M. Targeted stereology: sampling heterogeneously distributed
structures and lesions in the lung. American Journal of Physiology-Lung Cellular and Molecular
Physiology. 2024 Aug 1;327(2):L258-65.
https://doi.org/10.1152/ajplung.00321.2023 |
| 14 | Wang A, Ali A, Keshavjee S, Liu M, Cypel M. Ex vivo lung perfusion for donor lung assessment and
repair: a review of translational interspecies models. American Journal of Physiology-Lung Cellular
and Molecular Physiology. 2020 Dec 1;319(6):L932-40.
https://doi.org/10.1152/ajplung.00295.2020 |
| 15 | Susaki EA. Unlocking the potential of large-scale 3D imaging with tissue clearing techniques.
Microscopy. 2025 Jun 26;74(3):179-88.
https://doi.org/10.1093/jmicro/dfae046 |
| 16 | Xu X, Su J, Zhu R, Li K, Zhao X, Fan J, Mao F. From morphology to single-cell molecules:
high-resolution 3D histology in biomedicine. Molecular Cancer. 2025 Mar 3;24(1):63.
https://doi.org/10.1186/s12943-025-02240-x |
| 17 | Iber D. The control of lung branching morphogenesis. Current Topics in Developmental Biology. 2021
Jan 1;143:205-37.
https://doi.org/10.1016/bs.ctdb.2021.02.002 |
| 18 | Feng T, Cao J, Ma X, Wang X, Guo X, Yan N, Fan C, Bao S, Fan J. Animal models of chronic
obstructive pulmonary disease: A systematic review. Frontiers in Medicine. 2024 Oct 24;11:1474870.
https://doi.org/10.3389/fmed.2024.1474870 |
| 19 | Ochs M, Schipke J. A short primer on lung stereology. Respiratory Research. 2021 Nov 27;22(1):305.
https://doi.org/10.1186/s12931-021-01899-2 |
| 20 | Brenna C, Simioni C, Varano G, Conti I, Costanzi E, Melloni M, Neri LM. Optical tissue clearing
associated with 3D imaging: application in preclinical and clinical studies. Histochemistry and Cell
Biology. 2022 May;157(5):497-511.
https://doi.org/10.1007/s00418-022-02081-5 |
| 21 | Burri PH. Structural aspects of prenatal and postnatal development and growth of the lung. InLung
growth and development 2024 Nov 1 (pp. 1-36). CRC Press.
https://doi.org/10.1201/9781003574026-1 |
| 22 | Markel M, Ginzel M, Peukert N, Schneider H, Haak R, Mayer S, Suttkus A, Lacher M, Kluth D,
Gosemann JH. High resolution three‐dimensional imaging and measurement of lung, heart, liver, and
diaphragmatic development in the fetal rat based on micro‐computed tomography (micro‐CT). Journal of
Anatomy. 2021 Apr;238(4):1042-54.
https://doi.org/10.1111/joa.13355 |
| 23 | Ng WH, Johnston EK, Tan JJ, Bliley JM, Feinberg AW, Stolz DB, Sun M, Wijesekara P, Hawkins F,
Kotton DN, Ren X. Recapitulating human cardio-pulmonary co-development using simultaneous
multilineage differentiation of pluripotent stem cells. Elife. 2022 Jan 12;11:e67872.
https://doi.org/10.7554/eLife.67872 |
| 24 | Addis DR, Lambert JA, Ren C, Doran S, Aggarwal S, Jilling T, Matalon S. Vascular Endothelial
Growth Factor‐121 Administration Mitigates Halogen Inhalation‐Induced Pulmonary Injury and Fetal
Growth Restriction in Pregnant Mice. Journal of the American Heart Association. 2020 Feb
4;9(3):e013238.
https://doi.org/10.1161/JAHA.119.013238 |
| 25 | Myint MZ, Jia J, Adlat S, Oo ZM, Htoo H, Hayel F, Chen Y, Bah FB, Sah RK, Bahadar N, Chan MK.
Effect of low VEGF on lung development and function. Transgenic Research. 2021 Feb;30(1):35-50.
https://doi.org/10.1007/s11248-020-00223-w |
| 26 | Aydin E, Levy B, Oria M, Nachabe H, Lim FY, Peiro JL. Optimization of pulmonary vasculature
tridimensional phenotyping in the rat fetus. Scientific Reports. 2019 Feb 4;9(1):1244.
https://doi.org/10.1038/s41598-018-37906-8 |
| 27 | Chao CM, Moiseenko A, Kosanovic D, Rivetti S, El Agha E, Wilhelm J, Kampschulte M, Yahya F,
Ehrhardt H, Zimmer KP, Barreto G. Impact of Fgf10 deficiency on pulmonary vasculature formation in a
mouse model of bronchopulmonary dysplasia. Human molecular genetics. 2019 May 1;28(9):1429-44.
https://doi.org/10.1093/hmg/ddy439 |
| 28 | Warburton D. Conserved Mechanisms in the Formation of the Airways and Alveoli of the Lung.
Frontiers in Cell and Developmental Biology. 2021 Jun 15;9:662059.
https://doi.org/10.3389/fcell.2021.662059 |
| 29 | Lecarpentier Y, Gourrier E, Gobert V, Vallée A. Bronchopulmonary dysplasia: Crosstalk between
PPARγ, WNT/β-Catenin and TGF-β pathways; the potential therapeutic role of PPARγ agonists. Frontiers
in pediatrics. 2019 May 3;7:176.
https://doi.org/10.3389/fped.2019.00176 |
| 30 | Kiyokawa H, Morimoto M. Notch signaling in the mammalian respiratory system, specifically the
trachea and lungs, in development, homeostasis, regeneration, and disease. Development, Growth &
Differentiation. 2020 Jan;62(1):67-79.
https://doi.org/10.1111/dgd.12628 |
| 31 | Zepp JA, Morrisey EE. Cellular crosstalk in the development and regeneration of the respiratory
system. Nature reviews Molecular cell biology. 2019 Sep;20(9):551-66.
https://doi.org/10.1038/s41580-019-0141-3 |
| 32 | Song L, Li K, Chen H, Xie L. Cell cross-talk in alveolar microenvironment: from lung injury to
fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2024 Jul;71(1):30-42.
https://doi.org/10.1165/rcmb.2023-0426TR |
| 33 | Stoilova T, Ruhrberg C. Lung blood and lymphatic vascular development. Lung Stem Cells in
Development, Health and Disease (ERS Monograph). Sheffield, European Respiratory Society. 2021 Apr
1:31-43.
https://doi.org/10.1183/2312508X.10008920 |
| 34 | Kina YP, Khadim A, Seeger W, El Agha E. The lung vasculature: a driver or passenger in lung
branching morphogenesis?. Frontiers in Cell and Developmental Biology. 2021 Jan 14;8:623868.
https://doi.org/10.3389/fcell.2020.623868 |
| 35 | Shannon JM, Deterding RR. Epithelial-mesenchymal interactions in lung development. InLung growth
and development 2024 Nov 1 (pp. 81-118). CRC Press.
https://doi.org/10.1201/9781003574026-4 |
| 36 | Sutlive J, Xiu H, Chen Y, Gou K, Xiong F, Guo M, Chen Z. Generation, transmission, and regulation
of mechanical forces in embryonic morphogenesis. Small. 2022 Feb;18(6):2103466.
https://doi.org/10.1002/smll.202103466 |
| 37 | Ito JT, Lourenço JD, Righetti RF, Tibério IF, Prado CM, Lopes FD. Extracellular matrix component
remodeling in respiratory diseases: what has been found in clinical and experimental studies?.
Cells. 2019 Apr 11;8(4):342.
https://doi.org/10.3390/cells8040342 |
| 38 | Plosa EJ, Benjamin JT, Sucre JM, Gulleman PM, Gleaves LA, Han W, Kook S, Polosukhin VV, Haake SM,
Guttentag SH, Young LR. β1 Integrin regulates adult lung alveolar epithelial cell inflammation. JCI
insight. 2020 Jan 30;5(2):e129259.
https://doi.org/10.1172/jci.insight.129259 |
| 39 | Roman J. Cell-cell and cell-matrix interactions in development of the lung vasculature. InLung
growth and development 2024 Nov 1 (pp. 365-400). CRC Press.
https://doi.org/10.1201/9781003574026-13 |
| 40 | Crouch EC, Mecham RP, Davila RM, Noguchi A. Collagens and elastic fiber proteins in lung
development. InLung growth and development 2024 Nov 1 (pp. 327-364). CRC Press.
https://doi.org/10.1201/9781003574026-12 |
| 41 | Tongpob Y, Xia S, Wyrwoll C, Mehnert A. Quantitative characterization of rodent feto-placental
vasculature morphology in micro-computed tomography images. Computer Methods and Programs in
Biomedicine. 2019 Oct 1;179:104984.
https://doi.org/10.1016/j.cmpb.2019.104984 |
| 42 | Zakaria DM, Zahran NM, Arafa SA, Mehanna RA, Abdel-Moneim RA. Histological and physiological
studies of the effect of bone marrow-derived mesenchymal stem cells on bleomycin induced lung
fibrosis in adult albino rats. Tissue engineering and regenerative medicine. 2021 Feb;18(1):12741.
https://doi.org/10.1007/s13770-020-00294-0 |
| 43 | Debiane L, Reitzel R, Rosenblatt J, Gagea M, Chavez MA, Adachi R, Grosu HB, Sheshadri A, Hill LR,
Raad I, Ost DE. A design-based stereologic method to quantify the tissue changes associated with a
novel drug-eluting tracheobronchial stent. Respiration. 2019 Jul 15;98(1):60-9.
https://doi.org/10.1159/000496152 |
| 44 | Knudsen L, Brandenberger C, Ochs M. Stereology as the 3D tool to quantitate lung architecture.
Histochemistry and Cell Biology. 2021 Feb;155(2):163-81.
https://doi.org/10.1007/s00418-020-01927-0 |
| 45 | Sarabia-Vallejos MA, Ayala-Jeria P, Hurtado DE. Three-dimensional whole-organ characterization of
the regional alveolar morphology in normal murine lungs. Frontiers in Physiology. 2021 Dec
8;12:755468.
https://doi.org/10.3389/fphys.2021.755468 |
| 46 | Vasilescu DM, Phillion AB, Kinose D, Verleden SE, Vanaudenaerde BM, Verleden GM, Van Raemdonck D,
Stevenson CS, Hague CJ, Han MK, Cooper JD. Comprehensive stereological assessment of the human lung
using multiresolution computed tomography. Journal of Applied Physiology. 2020 Jun 1;128(6):1604-16.
https://doi.org/10.1152/japplphysiol.00803.2019 |
| 47 | Hussain S, Mubeen I, Ullah N, Shah SS, Khan BA, Zahoor M, Ullah R, Khan FA, Sultan MA. Modern
diagnostic imaging technique applications and risk factors in the medical field: a review. BioMed
research international. 2022;2022(1):5164970.
https://doi.org/10.1155/2022/5164970 |
| 48 | Deng Y, Rowe KJ, Chaudhary KR, Yang A, Mei SH, Stewart DJ. Optimizing imaging of the rat pulmonary
microvasculature by micro-computed tomography. Pulmonary circulation. 2019
Oct;9(4):2045894019883613.
https://doi.org/10.1177/2045894019883613 |
| 49 | Nicolas N, Dinet V, Roux E. 3D imaging and morphometric descriptors of vascular networks on
optically cleared organs. IScience. 2023 Oct 20;26(10).
https://doi.org/10.1016/j.isci.2023.108007 |
| 50 | Wu YC, Moon HG, Bindokas VP, Phillips EH, Park GY, Lee SS. Multiresolution 3D optical mapping of
immune cell infiltrates in mouse asthmatic lung. American Journal of Respiratory Cell and Molecular
Biology. 2023 Jul;69(1):13-21.
https://doi.org/10.1165/rcmb.2022-0353MA |
| 51 | Bucharskaya AB, Yanina IY, Atsigeida SV, Genin VD, Lazareva EN, Navolokin NA, Dyachenko PA,
Tuchina DK, Tuchina ES, Genina EA, Kistenev YV. Optical clearing and testing of lung tissue using
inhalation aerosols: prospects for monitoring the action of viral infections. Biophysical Reviews.
2022 Aug;14(4):1005-22.
https://doi.org/10.1007/s12551-022-00991-1 |
| 52 | Tielemans B, Marain NF, Kerstens A, Peredo N, Coll‐Lladó M, Gritti N, de Villemagne P, Dorval P,
Geudens V, Orlitová M, Munck S. Multiscale Three‐Dimensional Evaluation and Analysis of Murine Lung
Vasculature From Macro‐to Micro‐Structural Level. Pulmonary Circulation. 2025 Jan;15(1):e70038.
https://doi.org/10.1002/pul2.70038 |
| 53 | Borisova E, Lovric G, Miettinen A, Fardin L, Bayat S, Larsson A, Stampanoni M, Schittny JC,
Schlepütz CM. Micrometer-resolution X-ray tomographic full-volume reconstruction of an intact
post-mortem juvenile rat lung. Histochemistry and cell biology. 2021 Feb;155(2):215-26.
https://doi.org/10.1007/s00418-020-01868-8 |
| 54 | Badrou A, Mariano CA, Ramirez GO, Shankel M, Rebelo N, Eskandari M. Towards constructing a
generalized structural 3D breathing human lung model based on experimental volumes, pressures, and
strains. PLoS computational biology. 2025 Jan 13;21(1):e1012680.
https://doi.org/10.1371/journal.pcbi.1012680 |
| 55 | Ying X, Barlow NJ, Tatiparthi A. Micro-CT and volumetric imaging in developmental toxicology.
InReproductive and developmental toxicology 2022 Jan 1 (pp. 1261-1285). Academic Press.
https://doi.org/10.1016/B978-0-323-89773-0.00063-1 |
| 56 | Liang GB, He ZH. Animal models of emphysema. Chinese medical journal. 2019 Oct 20;132(20):2465-75.
https://doi.org/10.1097/CM9.0000000000000469 |
| 57 | Young AL, Bragman FJ, Rangelov B, Han MK, Galbán CJ, Lynch DA, Hawkes DJ, Alexander DC, Hurst JR.
Disease progression modeling in chronic obstructive pulmonary disease. American journal of
respiratory and critical care medicine. 2020 Feb 1;201(3):294-302.
|
| 58 | Yang Y, Di T, Zhang Z, Liu J, Fu C, Wu Y, Bian T. Dynamic evolution of emphysema and airway
remodeling in two mouse models of COPD. BMC Pulmonary Medicine. 2021 Apr 26;21(1):134.
https://doi.org/10.1186/s12890-021-01456-z |
| 59 | Greco F, Wiegert S, Baumann P, Wellmann S, Pellegrini G, Cannizzaro V. Hyperoxia-induced lung
structure-function relation, vessel rarefaction, and cardiac hypertrophy in an infant rat model.
Journal of translational medicine. 2019 Mar 18;17(1):91.
https://doi.org/10.1186/s12967-019-1843-1 |
| 60 | Ma Z, Tong S, Huang Y, Wang N, Chen G, Bai Q, Deng J, Zhou L, Luo Q, Wang J, Lu W. Development and
Characterization of a Novel Rat Model for Emulating Chronic Obstructive Pulmonary Disease-Associated
Cor Pulmonale. The American Journal of Pathology. 2025 May 1;195(5):831-44.
https://doi.org/10.1016/j.ajpath.2025.01.003 |
| 61 | Upadhyay P, Wu CW, Pham A, Zeki AA, Royer CM, Kodavanti UP, Takeuchi M, Bayram H, Pinkerton KE.
Animal models and mechanisms of tobacco smoke-induced chronic obstructive pulmonary disease (COPD).
Journal of Toxicology and Environmental Health, Part B. 2023 Jul 4;26(5):275-305.
https://doi.org/10.1080/10937404.2023.2208886 |
| 62 | Jia Z, Wang S, Yan H, Cao Y, Zhang X, Wang L, Zhang Z, Lin S, Wang X, Mao J. Pulmonary vascular
remodeling in pulmonary hypertension. Journal of Personalized Medicine. 2023 Feb 19;13(2):366.
https://doi.org/10.3390/jpm13020366 |
| 63 | Zhao J, Yang M, Wu X, Yang Z, Jia P, Sun Y, Li G, Xie L, Liu B, Liu H. Effects of paclitaxel
intervention on pulmonary vascular remodeling in rats with pulmonary hypertension. Experimental and
therapeutic medicine. 2019 Feb 1;17(2):1163-70.
|
| 64 | Jayasekera G, Wilson KS, Buist H, Woodward R, Uckan A, Hughes C, Nilsen M, Church AC, Johnson MK,
Gallagher L, Mullin J. Understanding longitudinal biventricular structural and functional changes in
a pulmonary hypertension Sugen-hypoxia rat model by cardiac magnetic resonance imaging. Pulmonary
Circulation. 2020 Jan;10(1):2045894019897513.
https://doi.org/10.1177/2045894019897513 |
| 65 | Bogaard HJ, Legchenko E, Chaudhary KR, Sun XQ, Stewart DJ, Hansmann G. Emphysema Is-at the
Most-Only a Mild Phenotype in the Sugen/Hypoxia Rat Model of Pulmonary Arterial Hypertension.
American Journal of Respiratory and Critical Care Medicine. 2019 Dec 1;200(11):1447-50.
https://doi.org/10.1164/rccm.201906-1200LE |
| 66 | Périz M, Pérez-Cano FJ, Rodríguez-Lagunas MJ, Cambras T, Pastor-Soplin S, Best I, Castell M,
Massot-Cladera M. Development and characterization of an allergic asthma rat model for
interventional studies. International Journal of Molecular Sciences. 2020 May 28;21(11):3841.
https://doi.org/10.3390/ijms21113841 |
| 67 | Savin IA, Zenkova MA, Sen'kova AV. Bronchial asthma, airway remodeling and lung fibrosis as
successive steps of one process. International journal of molecular sciences. 2023 Nov
7;24(22):16042.
https://doi.org/10.3390/ijms242216042 |
| 68 | Dos Santos TM, Righetti RF, Rezende BG, Campos EC, Camargo LD, Saraiva-Romanholo BM, Fukuzaki S,
Prado CM, Leick EA, Martins MA, Tibério IF. Effect of anti-IL17 and/or Rho-kinase inhibitor
treatments on vascular remodeling induced by chronic allergic pulmonary inflammation. Therapeutic
Advances in Respiratory Disease. 2020 Dec;14:1753466620962665.
https://doi.org/10.1177/1753466620962665 |
| 69 | Hall RD, Le TM, Haggstrom DE, Gentzler RD. Angiogenesis inhibition as a therapeutic strategy in
non-small cell lung cancer (NSCLC). Translational lung cancer research. 2015 Oct;4(5):515.
|
| 70 | Laskin DL, Malaviya R, Laskin JD. Role of macrophages in acute lung injury and chronic fibrosis
induced by pulmonary toxicants. Toxicological Sciences. 2019 Apr 1;168(2):287-301.
https://doi.org/10.1093/toxsci/kfy309 |
| 71 | Song LC, Chen XX, Meng JG, Hu M, Huan JB, Wu J, Xiao K, Han ZH, Xie LX. Effects of different
corticosteroid doses and durations on smoke inhalation-induced acute lung injury and pulmonary
fibrosis in the rat. International immunopharmacology. 2019 Jun 1;71:392-403.
https://doi.org/10.1016/j.intimp.2019.03.051 |
| 72 | Fragni D. Identification of novel readouts to assess anti-fibrotic efficacy of new compounds in a
bleomycin-induced pulmonary fibrosis mouse model.
|
| 73 | Kassab AA, Aboregela AM, Shalaby AM. Edaravone attenuates lung injury in a hind limb
ischemia-reperfusion rat model: a histological, immunohistochemical and biochemical study. Annals of
Anatomy-Anatomischer Anzeiger. 2020 Mar 1;228:151433.
https://doi.org/10.1016/j.aanat.2019.151433 |
| 74 | Zaghloul MS, Said E, Suddek GM, Salem HA. Crocin attenuates lung inflammation and pulmonary
vascular dysfunction in a rat model of bleomycin-induced pulmonary fibrosis. Life sciences. 2019 Oct
15;235:116794.
https://doi.org/10.1016/j.lfs.2019.116794 |
| 75 | Albanawany NM, Samy DM, Zahran N, El-Moslemany RM, Elsawy SM, Abou Nazel MW. Histopathological,
physiological and biochemical assessment of resveratrol nanocapsules efficacy in bleomycin-induced
acute and chronic lung injury in rats. Drug Delivery. 2022 Dec 31;29(1):2592-608.
https://doi.org/10.1080/10717544.2022.2105445 |
| 76 | Akil A, Gutiérrez-García AK, Guenter R, Rose JB, Beck AW, Chen H, Ren B. Notch signaling in
vascular endothelial cells, angiogenesis, and tumor progression: an update and prospective.
Frontiers in cell and developmental biology. 2021 Feb 16;9:642352.
https://doi.org/10.3389/fcell.2021.642352 |
| 77 | Zhang L, Tian Y, Zhao P, Jin F, Miao Y, Liu Y, Li J. Electroacupuncture attenuates pulmonary
vascular remodeling in a rat model of chronic obstructive pulmonary disease via the VEGF/PI3K/Akt
pathway. Acupuncture in Medicine. 2022 Aug;40(4):389-400.
https://doi.org/10.1177/09645284221078873 |
| 78 | Liu P, Gu Y, Luo J, Ye P, Zheng Y, Yu W, Chen S. Inhibition of Src activation reverses pulmonary
vascular remodeling in experimental pulmonary arterial hypertension via Akt/mTOR/HIF-1< alpha>
signaling pathway. Experimental cell research. 2019 Jul 1;380(1):36-46.
https://doi.org/10.1016/j.yexcr.2019.02.022 |
| 79 | Bishop D, Schwarz Q, Wiszniak S. Endothelial-derived angiocrine factors as instructors of
embryonic development. Frontiers in Cell and Developmental Biology. 2023 Jun 29;11:1172114.
https://doi.org/10.3389/fcell.2023.1172114 |
| 80 | Vallee A, Lecarpentier Y, Vallee JN. Interplay of opposing effects of the WNT/β-Catenin pathway
and PPARγ and implications for SARS-CoV2 treatment. Frontiers in immunology. 2021 Apr 13;12:666693.
https://doi.org/10.3389/fimmu.2021.666693 |
| 81 | Yan T, Shi J. Angiogenesis and EMT regulators in the tumor microenvironment in lung cancer and
immunotherapy. Frontiers in Immunology. 2024 Dec 16;15:1509195.
https://doi.org/10.3389/fimmu.2024.1509195 |
| 82 | Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative stress-responsive
microRNAs in heart injury. International Journal of Molecular Sciences. 2020 Jan 5;21(1):358.
https://doi.org/10.3390/ijms21010358 |
| 83 | Mikhael M, Makar C, Wissa A, Le T, Eghbali M, Umar S. Oxidative stress and its implications in the
right ventricular remodeling secondary to pulmonary hypertension. Frontiers in Physiology. 2019 Sep
24;10:1233.
https://doi.org/10.3389/fphys.2019.01233 |
| 84 | Liu WY, Wang L, Lai YF. Hepcidin protects pulmonary artery hypertension in rats by activating
NF-κB/TNF-α pathway. Eur Rev Med Pharmacol Sci. 2019 Jan 1;23(17):7573-81.
|
| 85 | Li Y, Ren W, Wang X, Yu X, Cui L, Li X, Zhang X, Shi B. MicroRNA-150 relieves vascular remodeling
and fibrosis in hypoxia-induced pulmonary hypertension. Biomedicine & Pharmacotherapy. 2019 Jan
1;109:1740-9.
https://doi.org/10.1016/j.biopha.2018.11.058 |
| 86 | Ji X, Yue H, Ku T, Zhang Y, Yun Y, Li G, Sang N. Histone modification in the lung injury and
recovery of mice in response to PM2 5 exposure. Chemosphere. 2019 Apr 1;220:127-36.
https://doi.org/10.1016/j.chemosphere.2018.12.079 |
| 87 | Wołowiec Ł, Mędlewska M, Osiak J, Wołowiec A, Grześk E, Jaśniak A, Grześk G. MicroRNA and lncRNA
as the future of pulmonary arterial hypertension treatment. International journal of molecular
sciences. 2023 Jun 4;24(11):9735.
https://doi.org/10.3390/ijms24119735 |
| 88 | Vachrushev NS, Shilenko LA, Karpov AA, Ivkin DY, Galagudza MM, Kostareva AA, Kalinina OV.
Differential Gene Expression in the Lungs of Rats with Experimental Chronic Thromboembolic Pulmonary
Hypertension. Journal of Evolutionary Biochemistry and Physiology. 2025 Jul;61(4):1025-38.
https://doi.org/10.1134/S0022093025040076 |
| 89 | Mathison M, Sanagasetti D, Singh VP, Pugazenthi A, Pinnamaneni JP, Ryan CT, Yang J, Rosengart TK.
Fibroblast transition to an endothelial "trans" state improves cell reprogramming efficiency.
Scientific Reports. 2021 Nov 19;11(1):22605.
https://doi.org/10.1038/s41598-021-02056-x |
| 90 | Avci E, Sarvari P, Savai R, Seeger W, Pullamsetti SS. Epigenetic mechanisms in parenchymal lung
diseases: bystanders or therapeutic targets?. International Journal of Molecular Sciences. 2022 Jan
4;23(1):546.
https://doi.org/10.3390/ijms23010546 |
| 91 | Hong J, Arneson D, Umar S, Ruffenach G, Cunningham CM, Ahn IS, Diamante G, Bhetraratana M, Park
JF, Said E, Huynh C. Single-cell study of two rat models of pulmonary arterial hypertension reveals
connections to human pathobiology and drug repositioning. American journal of respiratory and
critical care medicine. 2021 Apr 15;203(8):1006-22.
https://doi.org/10.1164/rccm.202006-2169OC |
| 92 | Hayati H, Feng Y, Hinsdale M. Inter-species variabilities of droplet transport, size change, and
deposition in human and rat respiratory systems: An in silico study. Journal of aerosol science.
2021 May 1;154:105761.
https://doi.org/10.1016/j.jaerosci.2021.105761 |
| 93 | Corley RA, Kuprat AP, Suffield SR, Kabilan S, Hinderliter PM, Yugulis K, Ramanarayanan TS. New
approach methodology for assessing inhalation risks of a contact respiratory cytotoxicant:
Computational fluid dynamics-based aerosol dosimetry modeling for cross-species and in vitro
comparisons. Toxicological Sciences. 2021 Aug 1;182(2):243-59.
https://doi.org/10.1093/toxsci/kfab062 |
| 94 | Yilmaz Y, Williams G, Walles M, Manevski N, Krähenbühl S, Camenisch G. Comparison of rat and human
pulmonary metabolism using precision-cut lung slices (PCLS). Drug metabolism letters. 2019 Apr
1;13(1):53-63.
https://doi.org/10.2174/1872312812666181022114622 |
| 95 | Boucherat O, Agrawal V, Lawrie A, Bonnet S. The latest in animal models of pulmonary hypertension
and right ventricular failure. Circulation Research. 2022 Apr 29;130(9):1466-86.
https://doi.org/10.1161/CIRCRESAHA.121.319971 |
| 96 | Hayati H, Feng Y. A precise scale-up method to predict particle delivered dose in a human
respiratory system using rat deposition data: An in silico study. InFrontiers in Biomedical Devices
2020 Apr 6 (Vol. 83549, p. V001T07A005). American Society of Mechanical Engineers.
https://doi.org/10.1115/DMD2020-9060 |
| 97 | Persson IM, Bozovic G, Westergren-Thorsson G, Enes SR. Spatial lung imaging in clinical and
translational settings. Breathe. 2024 Oct 1;20(3).
https://doi.org/10.1183/20734735.0224-2023 |
| 98 | Nizamoglu M, Joglekar MM, Almeida CR, Callerfelt AK, Dupin I, Guenat OT, Henrot P, Van Os L, Otero
J, Elowsson L, Farre R. Innovative three-dimensional models for understanding mechanisms underlying
lung diseases: powerful tools for translational research. European Respiratory Review. 2023 Jul
26;32(169).
https://doi.org/10.1183/16000617.0042-2023 |
| 99 | Mahmutovic Persson I, Falk Håkansson H, Örbom A, Liu J, von Wachenfeldt K, Olsson LE. Imaging
biomarkers and pathobiological profiling in a rat model of drug-induced interstitial lung disease
induced by bleomycin. Frontiers in Physiology. 2020 Jun 19;11:584.
https://doi.org/10.3389/fphys.2020.00584 |
| 100 | Umeda Y, Izawa T, Kazama K, Arai S, Kamiie J, Nakamura S, Hano K, Takasu M, Hirata A,
Rittinghausen S, Yamano S. Comparative anatomy of respiratory bronchioles and lobular structures in
mammals. Journal of Toxicologic Pathology. 2025;38(2):113-29.
https://doi.org/10.1293/tox.2024-0071 |
| 101 | Gui B, Wang Q, Wang J, Li X, Wu Q, Chen H. Cross-species comparison of airway epithelium
transcriptomics. Heliyon. 2024 Oct 15;10(19).
https://doi.org/10.1016/j.heliyon.2024.e38259 |
| 102 | Pennitz P, Kirsten H, Friedrich VD, Wyler E, Goekeri C, Obermayer B, Heinz GA, Mashreghi MF,
Büttner M, Trimpert J, Landthaler M. A pulmonologist's guide to perform and analyse cross-species
single lung cell transcriptomics. European Respiratory Review. 2022 Jul 27;31(165).
https://doi.org/10.1183/16000617.0056-2022 |
| 103 | Bauer C, Krueger M, Lamm WJ, Glenny RW, Beichel RR. lapdMouse: associating lung anatomy with local
particle deposition in mice. Journal of Applied Physiology. 2020 Feb 1;128(2):309-23.
https://doi.org/10.1152/japplphysiol.00615.2019 |
| 104 | Raredon MS, Adams TS, Suhail Y, Schupp JC, Poli S, Neumark N, Leiby KL, Greaney AM, Yuan Y, Horien
C, Linderman G. Single-cell connectomic analysis of adult mammalian lungs. Science advances. 2019
Dec 4;5(12):eaaw3851.
https://doi.org/10.1126/sciadv.aaw3851 |
| 105 | Shang Y. Numerical modelling of inhaled particle transport and deposition in human and rat nasal
cavities (Doctoral dissertation, RMIT University).
|
| 106 | Xie K, Ren B, Peng Y, Wang F, Zhang X, Wang J, Shu Q. Hypoxia-Primed Human Umbilical Cord
Mesenchymal Stem Cells Ameliorate Synovial Inflammation and Pulmonary Fibrosis via JNK/JAK-STAT3
Pathways in RA and RA-ILD. JAK-STAT3 Pathways in RA and RA-ILD.
|
| 107 | Aerts G, Willems L, Anthonissen R, De Jonghe B, Michiels J, Celen R, Verhaegen J, Vermaut A,
Geudens V, Hooft C, Kerckhof P. Micro-CT Based Approach to Quantitatively Evaluate Vascular Changes
in Pulmonary Arterial Hypertension. The Journal of Heart and Lung Transplantation. 2024 Apr
1;43(4):S114.
https://doi.org/10.1016/j.healun.2024.02.227 |
| 108 | Neelakantan S, Xin Y, Gaver DP, Cereda M, Rizi R, Smith BJ, Avazmohammadi R. Computational lung
modelling in respiratory medicine. Journal of The Royal Society Interface. 2022 Jun
8;19(191):20220062.
https://doi.org/10.1098/rsif.2022.0062 |
| 109 | Sisodia Y, Shah K, Sayyed AA, Jain M, Ali SA, Gondaliya P, Kalia K, Tekade RK. Lung-on-chip
microdevices to foster pulmonary drug discovery. Biomaterials Science. 2023 Jan 31;11(3):777-90.
https://doi.org/10.1039/D2BM00951J |
| 110 | Fawaz A, Ferraresi A, Isidoro C. Systems biology in cancer diagnosis integrating omics
technologies and artificial intelligence to support physician decision making. Journal of
Personalized Medicine. 2023 Nov 10;13(11):1590.
https://doi.org/10.3390/jpm13111590 |